Case Study: Preparing a Hospital’s Item Master to implement both a CloudBased ERP and Inventory Point-of-Use System
Symmetric used their data cleansing software and services to prepare an Academic Health System’s item master for simultaneous implementations of a cloud-based enterprise resource planning (ERP) software and inventory point-of-use (POU) system
Time to Value
In 7 days - Symmetric identified gaps in their item master as part of an assessment.
In 60 days - Symmetric delivered the following updates.
98,085 GTIN + packaging level updates
2,747 Duplicates resolved
40,918 Unique Descriptions updated
15,721 UNSPSCs code updates
60,751 HCPCS code additions
70,210 GMDN terms added
700 Latex indicators added
709 Bone/Tissue flags added
375 Sharps safety flags added
6,724 Implant flag updates
Maintenance
After delivering, Symmetric onboarded the Academic Health System’s team of 8 onto the Symmetric Terminal, where the team could look-up item data and access data maintenance reports to keep their Item Master prepared throughout the ERP and POU business transformation and implementation process.
The Problem
The supply chain team at the academic medical center was tasked with the challenge of preparing their item master’s data for a migration to the new ERP and to implement a POU inventory tracking system at the same time. Executing one transformation is already a challenge for operations teams but doing two at once is a large disruption requiring significant time. Due to time constraints, the academic medical center engaged Symmetric to overcome a number of challenges in preparing their item master for each system.
ERP Item Master preparation challenges:
Items must have a unique item description (Item Name)
Need to resolve item master duplicates
Need to resolve item description duplicates
Items must have a spend category assigned
Need to choose a spend category schema - like UNSPSC
Need to assign it to all Items
Once an Item’s packaging level has transactions, it cannot be edited
Need to clean and review Item master packaging
POU system Item Master preparation challenges:
Items must have GTINs (a type of UDI-compliant Barcode) assigned to their packaging levels in order for label scans to link to the corresponding Item packaging level in the POU system.
How we solved it
As the Symmetric team began onboarding and training users on the use of our application, our data scientists and medical device data experts employed our standard data cleansing and enrichment processes to improve customer data integrity upon initial upload. This enabled ongoing maintenance of this valuable data set in Symmetric’s application and all of the other tools and systems procured by the hospital, including their newly acquired ERP and POU systems.
The following steps were performed to achieve strong data quality:
Receipt of customer item master – The data set was produced using standard reporting methods from the current supply chain system and received via secure file transfer protocol. Once received by Symmetric, the necessary data transformation was performed to appropriately import it into the Symmetric Terminal.
Leveraged Symmetric’s proprietary matching technology to match items to our database – Upon import into the Terminal, Symmetric’s proprietary matching algorithm went to work to match this data set to our 14 million item database with over 400 data attributes per device.
Generated automated reports – Core reporting capabilities within Symmetric were utilized to:
Assess barcode data quality, identify errors, and recommend new barcodes
Assess UNSPSC code data quality, identify errors, and recommend new UNSPSC codes
Assess HCPCS data quality, identify errors, and recommend new HCPCS codes
Identify and verify duplicate item entries
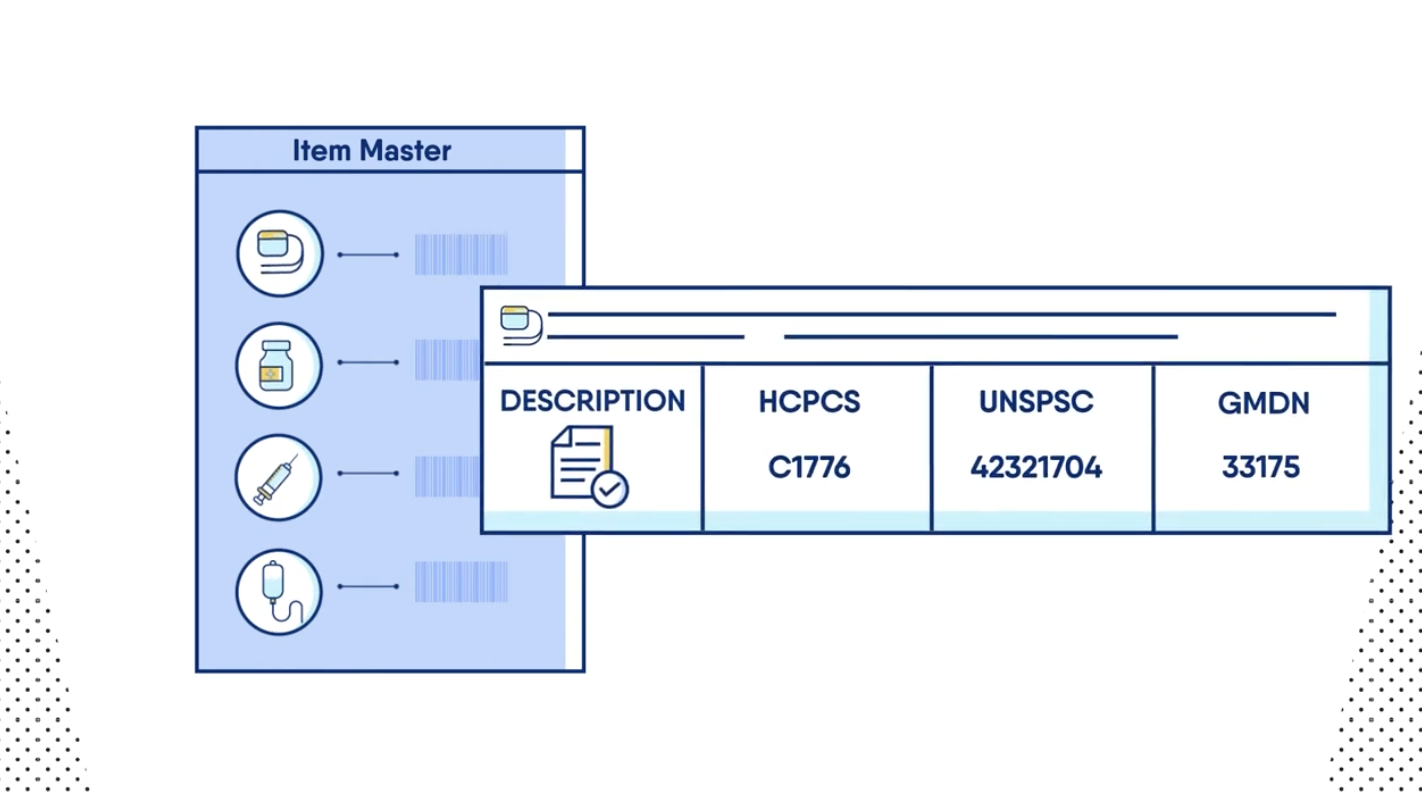
Defined core enrichment fields – The hospital and Symmetric team members defined core fields that would be updated using Symmetric data. The following fields were determined to be essential to hospital operations.
Description
Unit of Measure
UNSPSC (United Nations Standard Products and Services Code)
GTINs (Global Trade Item Numbers)
Manufacturer Name
Manufacturer Catalog Number
Latex Flag
Bone/Tissue Flag
Sterile Flag
Implant Flag
Validated proposed changes – A report including all item data and the proposed changes were prepared for review. Proposed changes were then reviewed and approved or additional feedback was provided.
Upon validation, the final report of Symmetric enriched item data was provided to the customer who performed updates to their item master in batch.
Looking forward
Symmetric continues to partner with Supply Chain and Operations Data leaders to shorten the gap to implement the vision of the UDI program - bringing up-to-date medical device data to the healthcare supply chain.
We are integrating into the item add/change workflows of many ERP systems, supporting nomenclature mapping relations projects with global regulatory and standards organizations, and supporting the accurate scanning of barcodes for traceability and patient safety.